
Position Your DHT Innovation for Success

|
Written by Christine Guo, PhD |
Clinical trialists today can benefit from the many hard lessons learned over the last 10 years. People new to this space tend to focus on the appearance and features of the wearable devices, often drawing on their own experiences as consumers. In reality, successful use of wearables in clinical trials has more to do with the good-and-old clinical trial practices, such as operations, protocol adherence, and record retention.
In my previous blog post, we covered adoption, endpoint positioning, and regulatory guidance for accelerating clinical development with patient-centered digital endpoints. Here, we will briefly touch on three additional factors impacting success of digital health technology (DHT) trials.
Digital Health Operations
Global multi-center clinical trials place a high demand on the operational excellence of DHT deployment. An experienced operations team is essential to ensure proper DHT use, minimal missing data, and high quality data collection. A thorough qualification process should be in place to ensure that the DHT provider has adequate SOPs, quality and compliance management processes, a mature logistics workflow, proper site training, and patient-facing materials adapted to the local language and culture. A clear coordination and communication plan between the site and the DHT provider is also critical to ensure timely responses to technical issues and adherence monitoring.
After iterations and improvements over hundreds of trials, DHTs are now routinely deployed in large scale global trials. In our experience, while North American and European countries host the majority of DHT studies, DHT deployments to South American, Australia, and Asian countries are on the rise (Fig. 1). Learn more about the best practice of DHT operations in this recent webinar.
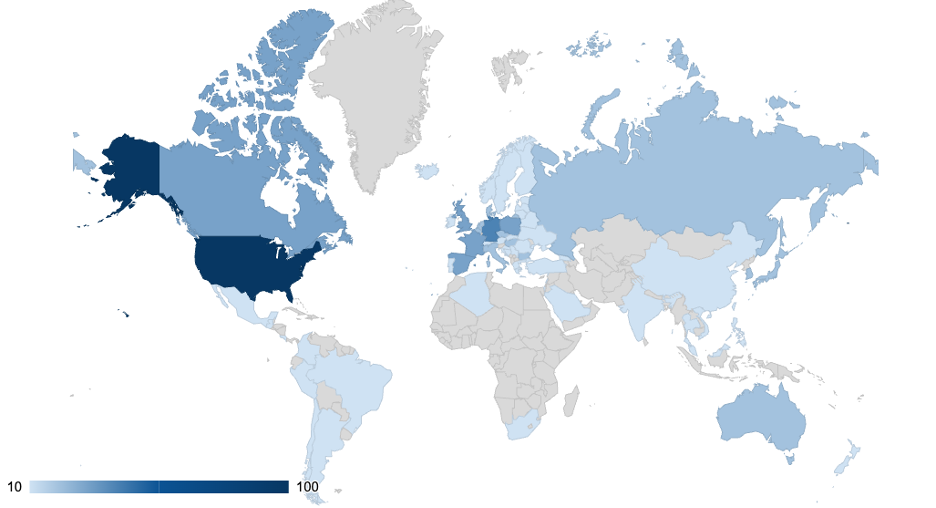
Fig. 1 - Percentage of studies with wearable data across geographic areas
Sensor Data Analytics
Sensor data collected from wearable devices are multi-dimensional, high frequency, big data, requiring processing steps of calibration, filtering, dimension reduction, and feature extraction before submitting into statistical analyses for trial insights. These data processing steps required to analyze DHT data can be foreign and confusing to study teams that primarily work with simple calculations used for scales and questionnaires. On the other hand, engineers and data scientists who are experts with digital data processing might not understand the requirements for clinical trial analytics.
This gap in data analytics has been one of the biggest barriers to the effective use of DHT data in clinical trials. The good news is that both DHT providers and sponsors have been developing multiple-disciplinary expertise to connect the dots from sensor data to clinical meaningfulness. Many scientific and analytical services are available nowadays to assist study teams in their quest to find new treatments and therapies.
It remains crucial to plan analytical resources properly during the study planning stage. A common mistake in the early days was that well-intentioned study teams designed DHTs into the study protocols and successfully collected digital data, but then found themselves with no plan to unleash its real value for clinical insights. We encourage study teams to consider the unique analytical challenges associated with DHT data and allocate proper analytical resources from the get-go.
Digital Health Operations
Through the last 20 years supporting clinical research, we have learned that an essential requirement for using sensor-based DHTs in clinical investigation is the collection and retention of raw sensor data. This consideration, unfortunately, tends to be overlooked by many study teams.
To illustrate this point, let’s draw an analogy to the use of patient reported outcomes (PROs) and clinician reported outcomes (ClinROs) in clinical investigation (Fig. 2). In the cases of PROs/ClinROs, the items in the questionnaires are the source data and required by regulatory agencies so they can be reviewed for discrepancies. They can be further mined in the future for subcategories or individual items to gain additional insights. In the case of wearable devices, the raw sensor data is the source data and should be retained for the same reason as in the case of PROs and ClinROs.
Furthermore, compared to the scoring criteria for PRO/ClinRO, the processing steps (algorithms) used to convert raw sensor data to digital measures are substantially more complex. The improvements to algorithms are also evolving at a much faster pace than typical clinical development. In the case of consumer devices, they might even be altered by firmware updates that impact the consistency of digital measures during the trial. Without the retention of the raw sensor data, study teams are at risk of suboptimal and/or inconsistent data processing, leading to permanent data loss.
To maximize the investment in DHTs, we highly advise study teams to require raw sensor data for record retention. This is the best practice to ensure data quality and traceability in regulated trials, to keep pace with state-of-the-art digital science techniques, and to ensure compatibility across studies, thus maximizing their scientific impact. Interested readers can find more details in this white paper.
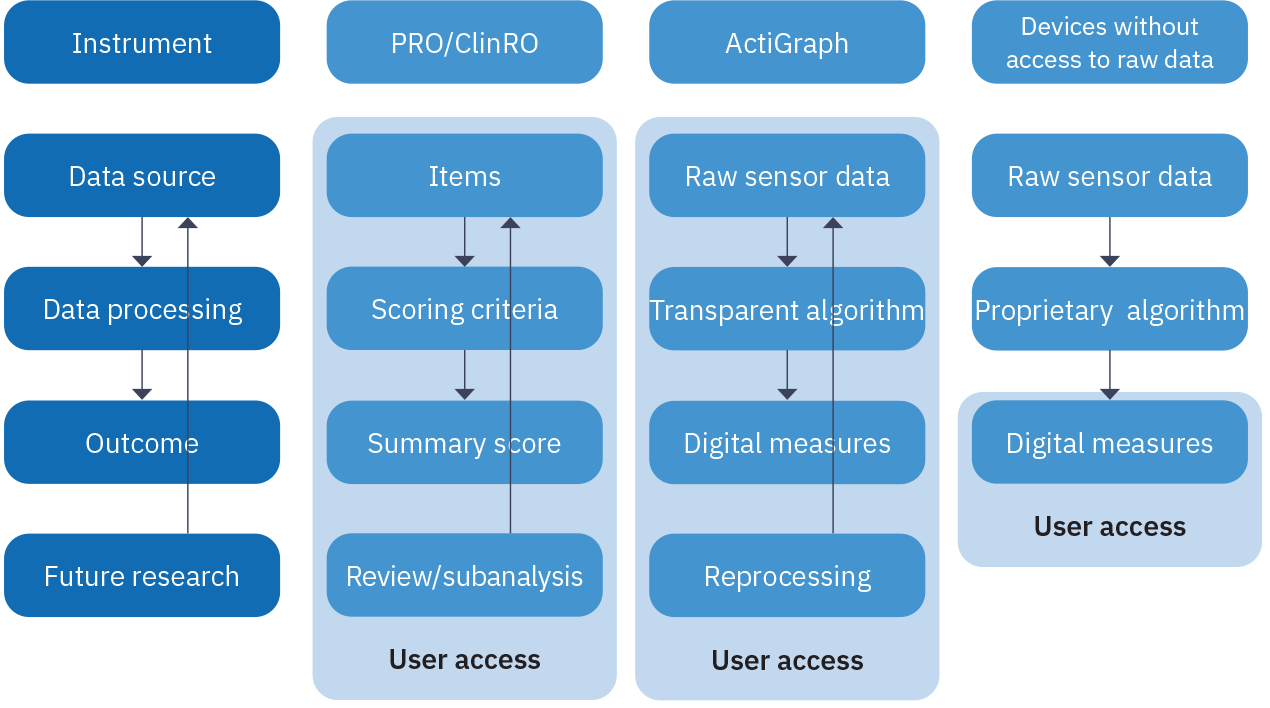
Fig. 2
Change does not happen quickly in the clinical trial industry. Over the last 10 years, the industry has been exploring the use of wearable devices and gaining valuable experience in the process. With increasing pressure on the efficiency and productivity of clinical development in the post-pandemic era, now is the time to embrace the opportunities brought by wearable devices.
Clinical trial sponsors can benefit from partnering with an experienced DHT provider with a fully integrated solution over the entire digital data value chain. As FDA noted in their DHT guidance, collaboration between sponsors and DHT manufacturers could be key to successfully leverage DHT data to accelerate clinical development. For more details, please see “What Does the New FDA DHT Guidance Mean? A Practical Guide for Sponsors.”
Contact ActiGraph to discuss your clinical development challenges.