In the evolving landscape of clinical trials, wearable digital health technologies give clinical researchers the capability of continuous, real-life monitoring of participant behavior. This data offers an objective and reliable assessment of meaningful aspects of health that can provide important insights into treatment efficacy and advance therapeutic developments.
One critically important aspect of realizing the value of wearable data in clinical trials is maximizing patient adherence. There are best practices clinical researchers can follow to maximize adherence of wearables in clinical trials, such as selecting a fit-for-purpose device for your study, avoiding optionality of wearable endpoints in the study, optimizing wear time in your protocol, and monitoring adherence.
Even with optimal device specifications and protocol design, there will still be instances of low-adherence. It’s ideal that adherence monitoring be near real-time, because discovering too late that data is missing can initiate a cascade of issues that are difficult for data managers to reconcile. Missing data could affect data quality in several ways, including reducing statistical power and introducing statistical biases, ultimately making it difficult for biostatisticians to compute the desired clinical endpoint.
Recognizing the need for better adherence monitoring capabilities that allow clinical researchers to quickly identify and correct non-adherence, ActiGraph is excited to introduce its latest solution – ActiTrack.
ActiTrack is a near real-time adherence monitoring solution for clinical trial sponsors. It compares the wearable device data collected with the schedule of activities and visit dates during an individual's participation in the trial.
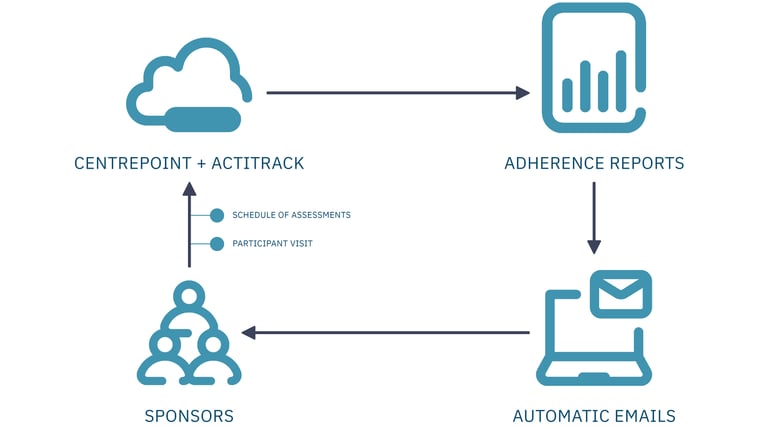
Figure 1 - ActiTrack Workflow
ActiTrack generates a custom report on each individual participant's adherence and sends it to a predefined list of study team members. This enables clinical teams to detect near real-time adherence issues and act quickly with effective mitigation strategies, such as sending participant reminders, replacing malfunctioning devices, fixing data transfer connections, and/or providing site staff with additional training and resources.
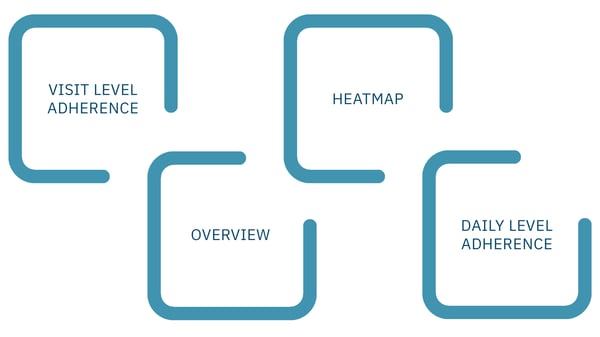
Figure 2: ActiTrack's custom report provides an overview of participant adherence with a clear call to action.
Additional ActiTrack features currently in development include automatic adherence alerts sent directly to the site, and an adherence prediction feature that can inform study design and highlight study timepoints that may require additional attention.
To learn more about using ActiTrack in your clinical trial, reach out to your ActiGraph account manager or contact us today.